
The ICHOM Set of Patient-Centered Outcome Measures for Lung Cancer is the result of hard work by a group of leading physicians, measurement experts and patients. It is our recommendation of the outcomes that matter most to patients with Lung Cancer. We urge all providers around the world to start measuring these outcomes to better understand how to improve the lives of their patients.
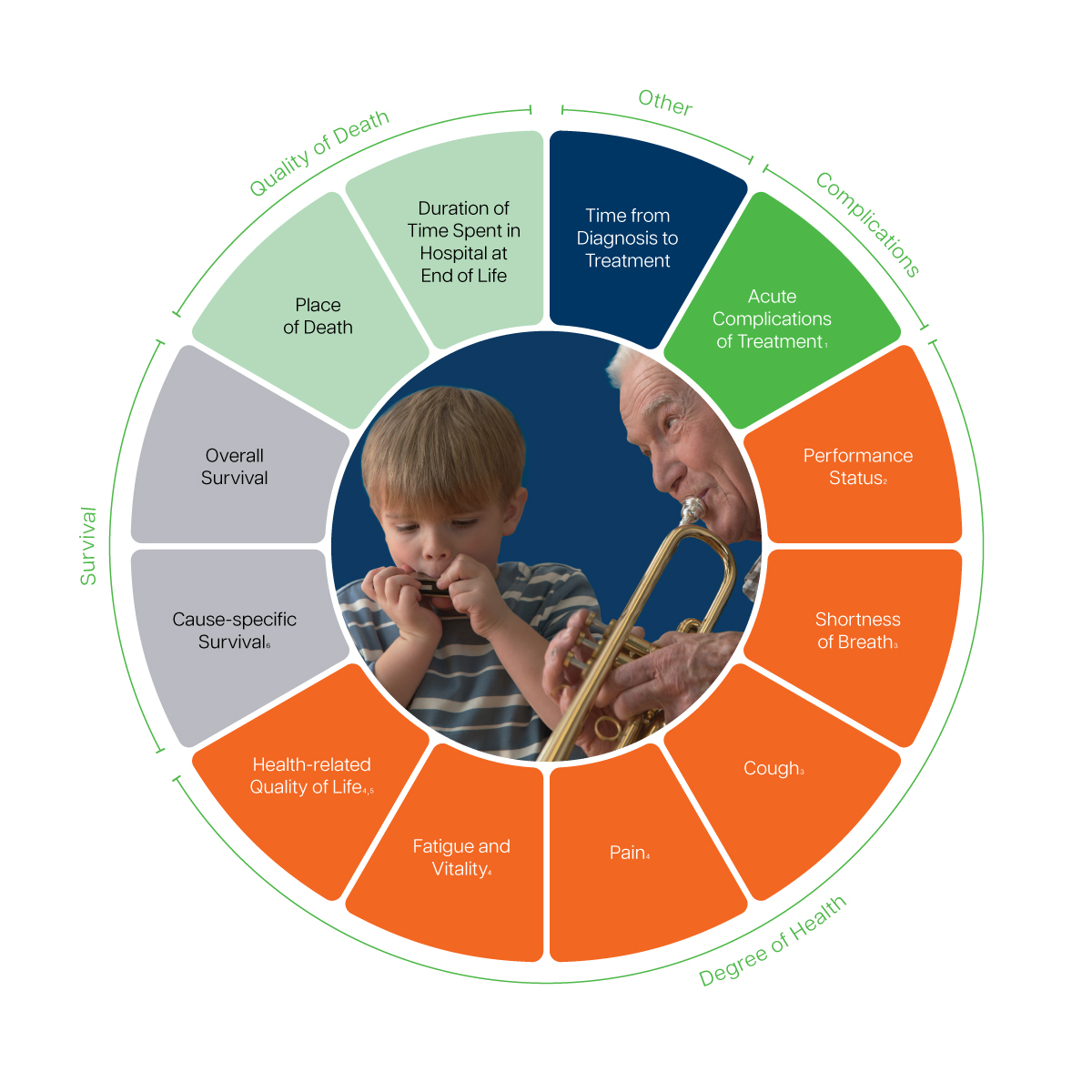
- Includes major surgical complications, major radiation complications, and major systemic therapy complications. Recorded via the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0
- Recorded via the Eastern Cooperative Oncology Group (ECOG) score
- Recommended to track via the European Organization for Research and Treatment of Cancer Quality of Life Lung Cancer-Specific Questionnaire (EORTC QLQ-LC13)
- Recommended to track via the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30)
- Includes physical, emotional, cognitive, and social function and well-being 6 Includes treatment-related mortality and cause of death
- Includes treatment related mortality and cause of death

All of the basic information you need to implement ICHOM Sets is available in the links above. However, for a faster, more easily integrated process, we offer digitised versions of the Set measures. These are designed for immediate upload to existing EMR systems. This is available as part of our Accreditation package, which recognises your work in putting patient outcomes at the heart of treatment.
Team that developed this set
AUSTRALIA
Robert Stirling | Monash University
BELGIUM
Jan van Meerbeeck | Antwerp University Hospital
BRAZIL
Clarissa Baldatto | Clínicas Oncológicas Integradas
NETHERLANDS
Franz Schramel | St. Antonius Hospital
Suresh Senan | VU University Medical Centre Amsterdam
Michel Wouters | Netherlands Cancer Institute
UNITED KINGDOM
Matthew Baker*
David Baldwin | Nottingham University Hospitals
Diana Borthwick | Edinburgh Cancer Research Centre
Jesme Fox | Roy Castle Lung Cancer Foundation
Tom Haswell*
Mick Peake | University Hospital Leicester
UNITED STATES
Janet Abrahm | Dana-Farber Cancer Institute
David Carbone | Ohio State University Comprehensive Cancer Center
Aileen Chen | Dana-Farber Cancer Institute
Marianna Koczywas | City of Hope National Medical Center
Benjamin Kozower | University of Virginia Health System
Kimberley Mak | Dana-Farber Cancer Institute
Reza Mehran | MD Anderson Cancer Center
* Parent representative
Are you implementing ICHOM Sets?
If your are implementing ICHOM Sets, please help us understand more about your journey by filling in our Implementation Survey. Click on the link below to complete:
Implementation Map
We would like to add you to our Implementation Map if you are implementing or have implemented ICHOM Sets. Please click on the button below for more information.





